Improving granularity
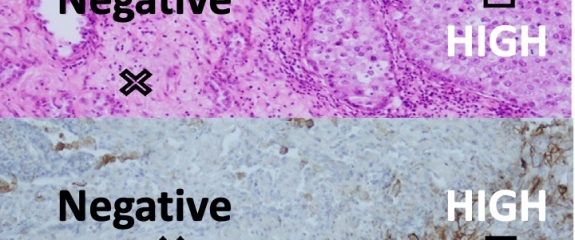
Intratumoural heterogeneity of pulmonary adenocarcinoma challenges accurate interpretation of PD-L1 immunohistochemistry, the only indicator for successful anti-PD-L1 immunotherapy. Researchers have shown that PD-L1 evaluation needs better granularity.
Non-small cell lung cancers (NSCLC) are a heterogeneous group of tumors that differ in their histological patterns, biological behavior and molecular characteristics. Non-small cell lung cancer (NSCLC) adenocarcinomas usually demonstrate intratumoral heterogeneity reflecting the presence of various histological patterns in individually different proportions. Immunotherapy has emerged as a promising treatment modality for patients with advanced NSCLC. Immune checkpoint inhibitors targeting the programmed death ligand 1 (PD-L1) signaling pathway to reactivate suppressed immune systems are serving to improve prognosis of lung cancer patients. Checkpoint inhibitors currently approved for clinical use include the anti-PD-1 agents nivolumab and pembrolizumab and the anti-PD-L1 agents atezolizumab and avelumab. However, clinical response to checkpoint inhibitors is unpredictable and could relate to the heterogenous nature of PD-L1 expression in tumours. This study investigated immunohistochemical expression of PD-L1 and its potential for predicting the efficacy of immunotherapy in NSCLC patients.
Adenocarcinomas with high intratumoural heterogeneity were categorised as ‘mixed adenocarcinomas’. PD-L1 expression was determined immunohistochemically using tumour proportion scores (TPS). In ‘mixed adenocarcinomas’ PD-L1 scores were assessed across tumour areas with specific histological patterns. Comparisons were performed between histologically distinct differentiated tumours and/or histological areas. Poorly differentiated adenocarcinomas, represented by predominantly solid or micropapillary histological patterns, showed significantly higher expression of PD-L1 than other subtypes (p<0.001). Differentiation of intraadenocarcinoma components was inversely correlated with PD-L1 expression: there were more PD-L1 positive cells in poorly differentiated areas than less differentiated (p<0.001), or than well differentiated areas (p<0.001), and in less differentiated more than well differentiated areas (p=0.001).
The authors cconcluded thst PD-L1 expression is associated with poorly differentiated morphology in adenocarcinomas with intratumoural histological heterogeneity. Consequently, a TPS approach may not account for the contribution of more aggressive tumour components with higher levels of PD-L1 expression in within the tumour. Performing spectral analyses of PD-L1 expression across tumours is likely to be more accurate.


